We specialise in taking new drugs from their nascent development stages to first in man clinical studies, and support technology transfer to CMOs for large scale commercial manufacture.
We provide both development support and GMP manufacture of finished dosage forms, from our state of the art laboratories situated at the Loughborough University Science and Enterprise Park, in Loughborough, UK.
Expertise & Facilities
• Comprehensive pharmaceutical development program all undertaken in our state-of-the-art in-house laboratories in Loughborough, UK
• In-house aseptic production and terminal sterilisation capabilities
• Rapid proof of concept clinical product development with In-house ICH stability chambers
• Investigational medicinal product manufacture: gels/semi-solids, solid injectables, liquid injectables, transdermal and Microneedle patches, in our GMP aseptic and non-aseptic Manufacture suite
Manufacture Capabilities
-
Capsules (Non-GMP only)
Small and medium scale capsule fill operation.
-

Injectables
We can fill up to 2000 cartridges per hour using our cartridge filling line for injectables for use with pen injectors.
-

Tabletting (Non-GMP only)
We can produce up to 2000 tablets per hour.
-

Transdermal Patch
Our transdermal coating line allows the production of a few dozen to several thousand patches for clinical evaluation .
-

Ampoules, vials, PFS and cartridges
-
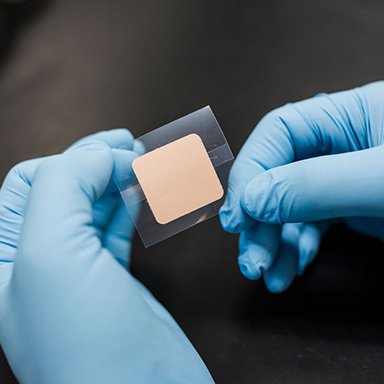
Microneedle Patches


